Click Here for Answer
Lava Lamp
-
Time Required: 0-30 Minutes
-
Materials Needed
-
1 L (32 oz.) Plastic Bottle with Screw Cap
-
Food Coloring (as needed)
-
12 oz. (1 1/2 cups) Water
-
16 oz. (2 cups) Vegetable Oil
-
One Effervescent Antacid Tablet
(Alka-Seltzer)
-
-
Optional Materials: Flashlight (flashlight on a cell phone also works)
-
Concepts: Acid/Base, Chromatography, Density
-
Parental Supervision: Recommended for children under 12
-
Safety: Effervescent antacid tablets contain aspirin and parental supervision should be present for children under the age of 12, as these tablets should not be consumed, unless these are used for their desired purpose.
Procedure
-
Remove the label from an empty 1 L (32 oz.) bottle
-
Add water until the bottle is about 1/3 full (about 12 oz. or 1 1/2 cups)
-
Add food coloring into the bottle and shake to make the color of the water uniform.
-
Repeat step 3 until the desired color is obtained.
-
Fill the bottle the rest of the way with vegetable oil (about 16 oz. or 2 cups) until about 3 inches from top.
-
Break the effervescent antacid tablet into pieces and slowly add some of the pieces into the bottle.
-
Let the tablet pieces sink into the water, bubbles should immediately develop, causing the colored water to move through the oil layer
-
A single tablet will last XX minutes, additional tablets (one at a time) can be added after the bubbling stops.
Optional
-
The bottle can be placed on top of flashlight in a dark room for a "lava lamp" effect
What is going on?
Water and vegetable oil are two liquids that do not mix (immiscible) when added together. The density of water (1.0 g/mL) is greater (heavier per a given volume) than the density of vegetable oil (~0.93 g/mL) leading to the water layer being at the bottom of the bottle.
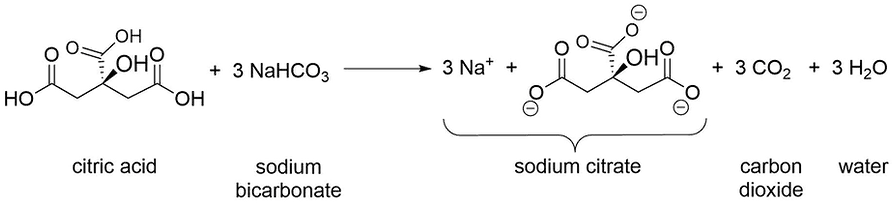
An effervescent antacid tablet contains aspirin, sodium bicarbonate, and citric acid. When the effervescent antacid tablet mixes with water, the citric acid dissolves and reacts with sodium bicarbonate to generate carbon dioxide (CO2) and sodium citrate.
The formed carbon dioxide gas produces bubbles, which carry small amounts of water and food coloring with it as the bubbles rise through the vegetable oil. Once the bubble reaches the top of the vegetable oil, the bubble breaks, releasing the gas and causing the colored water to sink through the vegetable oil until the colored water rejoins the water layer.
Clean Up
Upon completion of the experiment, the oil and water mixture can be flushed down the toilet or poured down the drain, followed by running hot water for two minutes.
Click Here for Answer to Disappear
Discussion Questions
-
When ice is added to a glass of water, why does the ice float to the top?
-
What does it mean for a compound to be immiscible?
-
In this experiment, citric acid reacts with sodium bicarbonate to generate carbonic acid (H2CO3). Why is carbon dioxide observed instead of carbonic acid?